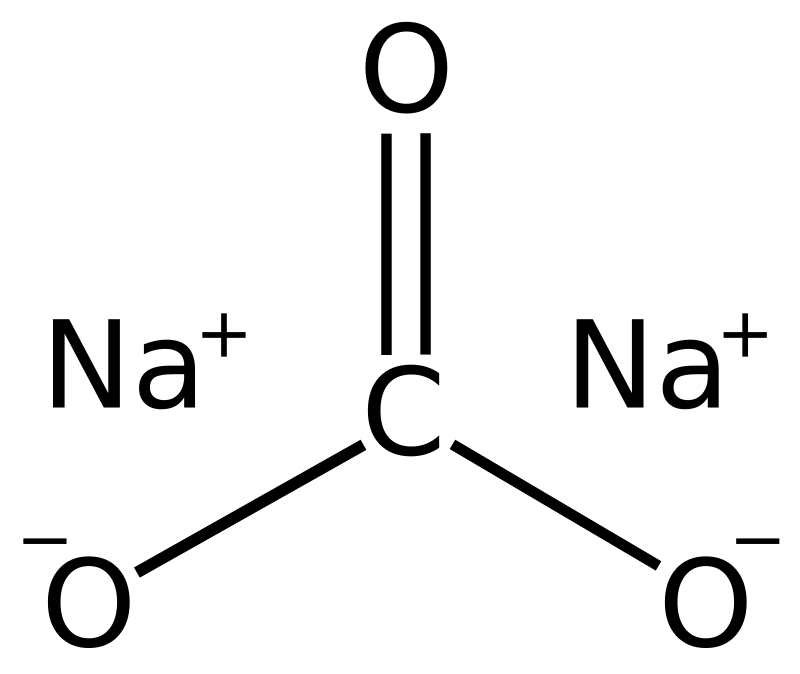
In a sample of sodium carbonate, some sodium sulphate is mixed 2.50 g of this sample is dissolved and the volume made up to 500 mL. 25 mL of this solution neutralises

Calculate the quantity of sodium carbonate (anhydrous) required to prepare 250mL `(M)/(10)` - YouTube

A mixture of sodium chloride (NaCI) and anhydrous sodium carbonate (Na,cO,) has a mole ratio 2:1. It is dissolved in water and treated with BaCl2 solution. The mass of BaCO, precipitated is

Please solve 40,41,42 37 38 39 40 41 42 43 Determine the percentage - Chemistry - Some Basic Concepts of Chemistry - 12637943 | Meritnation.com

What is the formula mass of anhydrous sodium carbonate ? (Given that the atomic masses of sodium... - YouTube

Please solve 40,41,42 37 38 39 40 41 42 43 Determine the percentage - Chemistry - Some Basic Concepts of Chemistry - 12637943 | Meritnation.com

Conversion of gaseous effluents of power plant to sodium carbonate: A value-added material for powder detergent - ScienceDirect

Speci…c heat capacity for the anhydrous sodium carbonate. The inset... | Download Scientific Diagram
Determine tge percentage composition of a mixture of anhydrous sodium carbonate and sodium bicarbonate from the following data : weight of mixture taken = 2g loss in weight on heating = 0.11