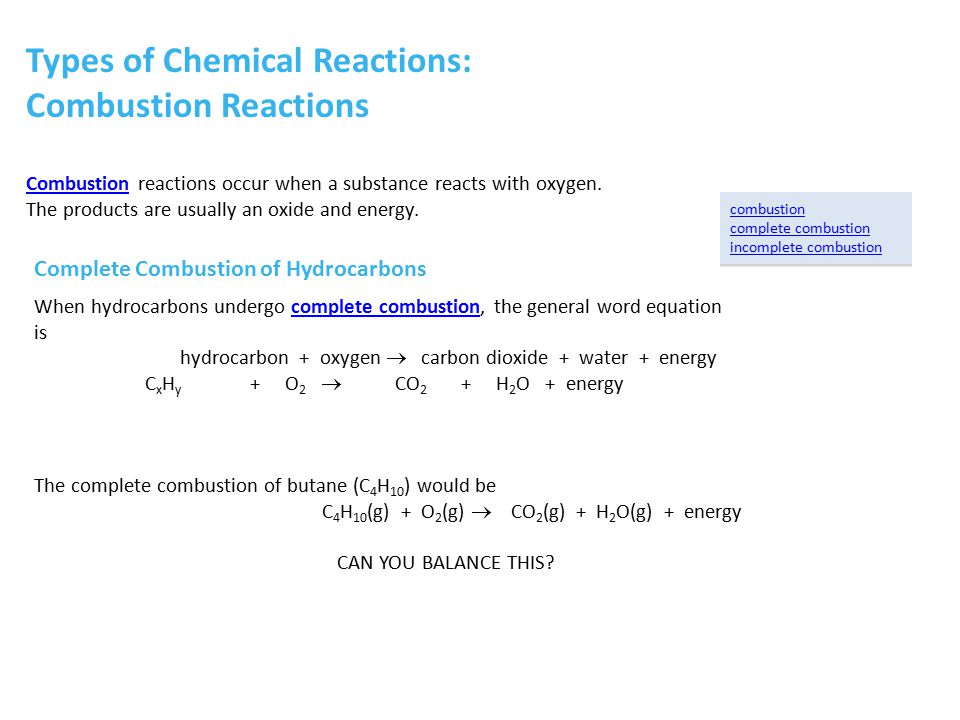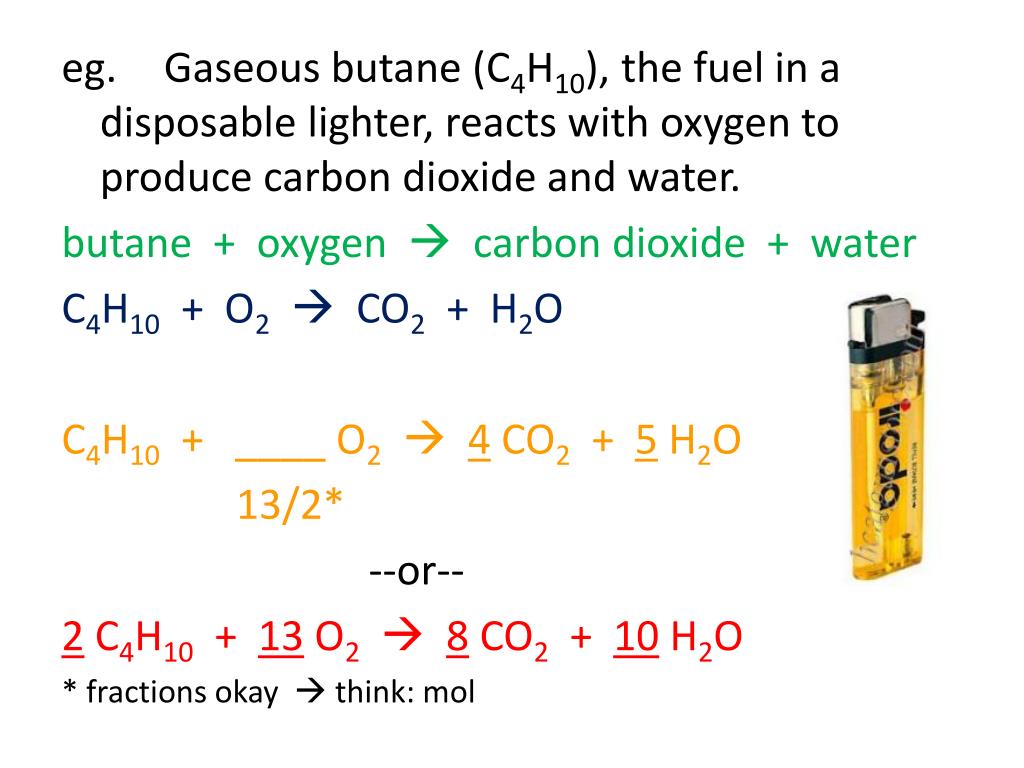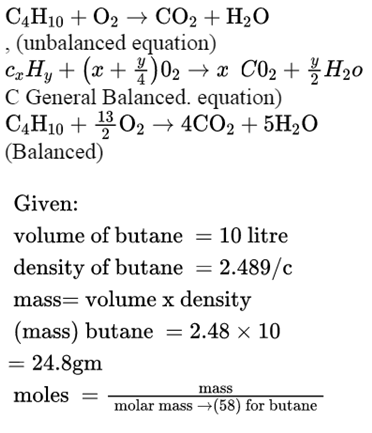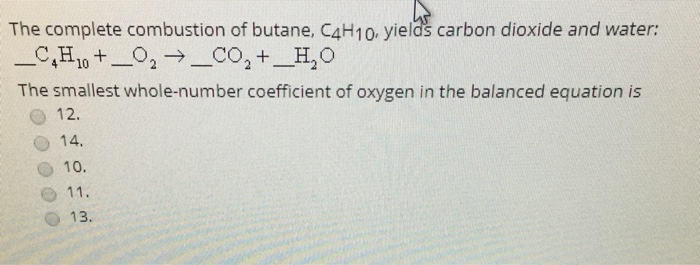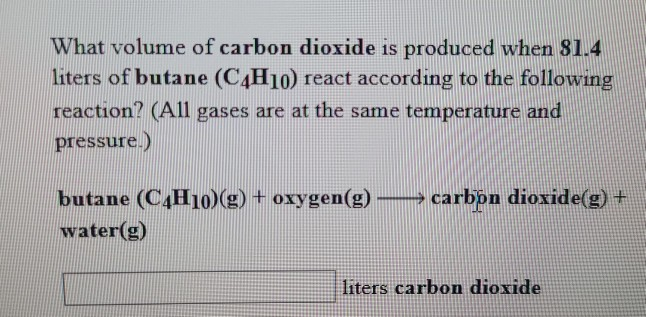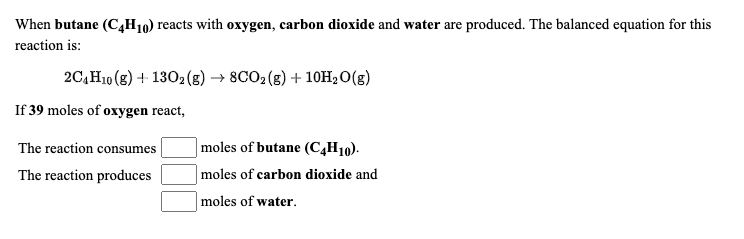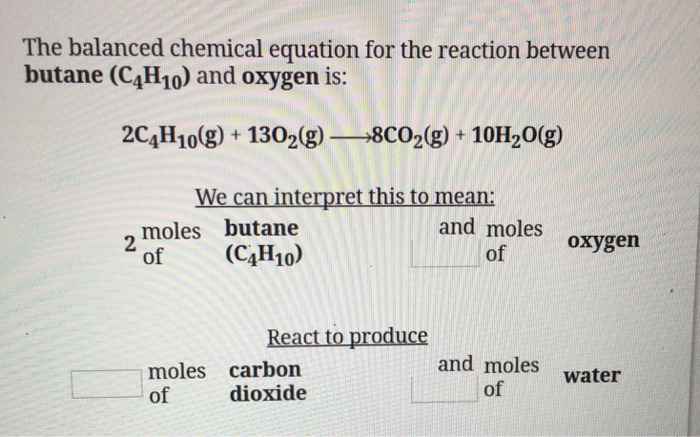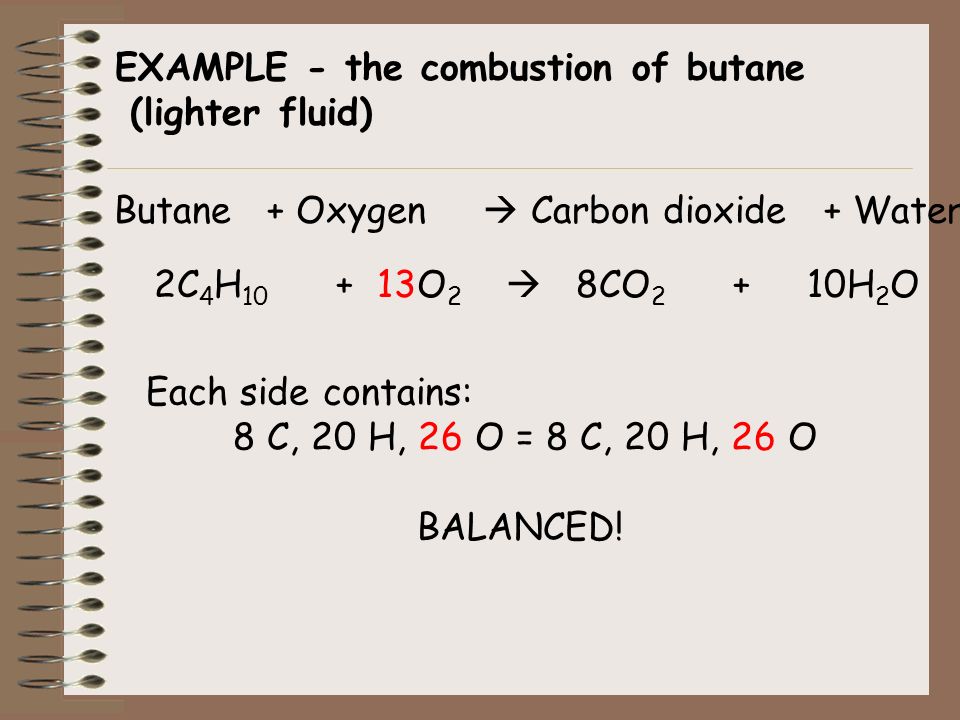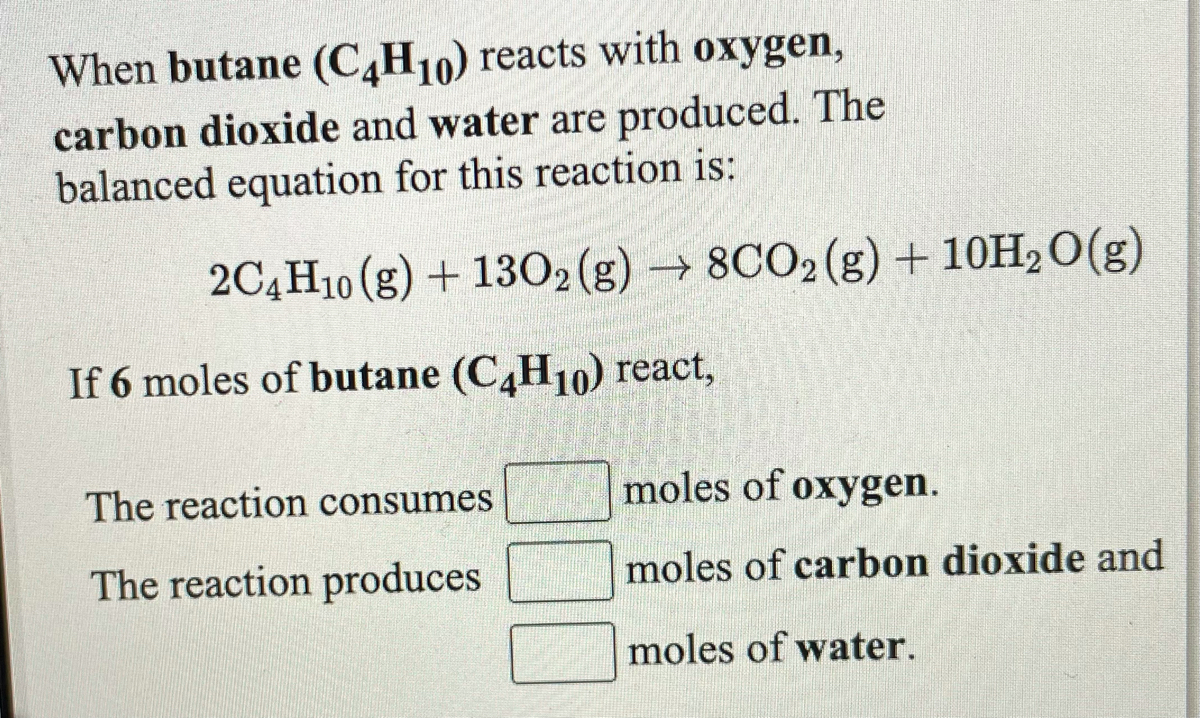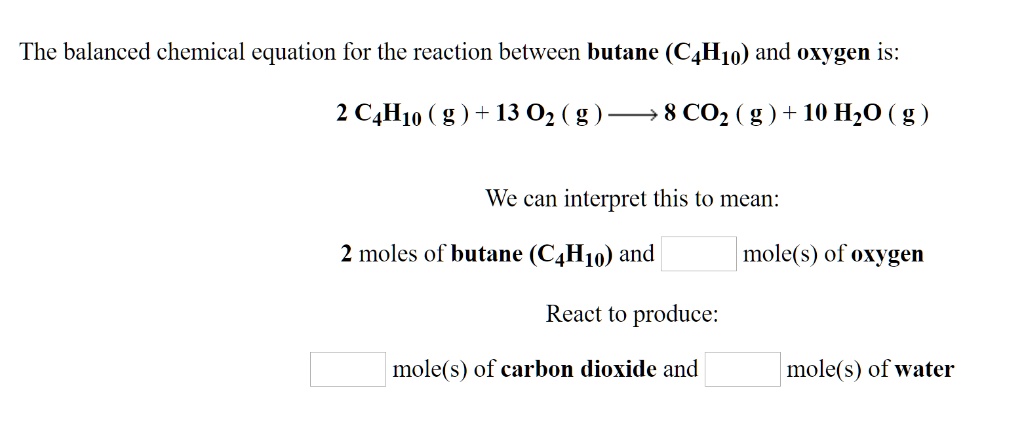
SOLVED: The balanced chemical equation for the reaction between butane (C4H1o) and oxygen is: 2 C4H1o ( g ) + 13 02 ( g ) 8C02 ( g +10 Hz0 ( g )
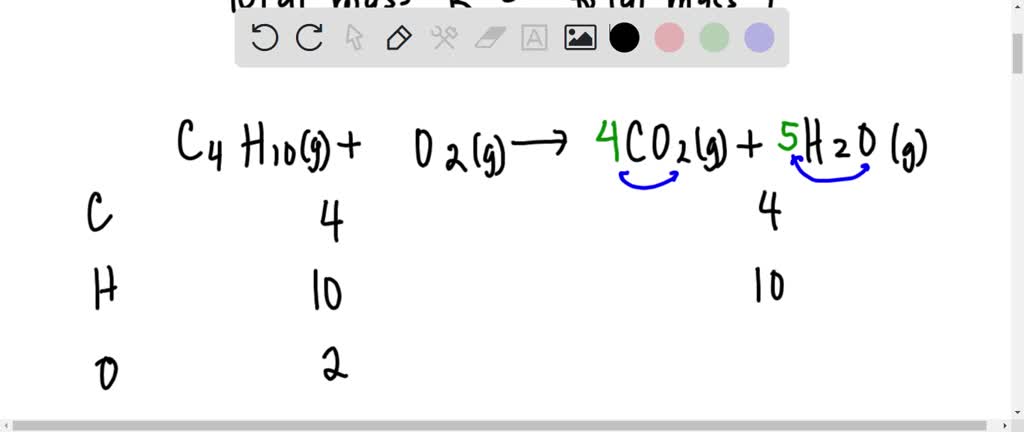
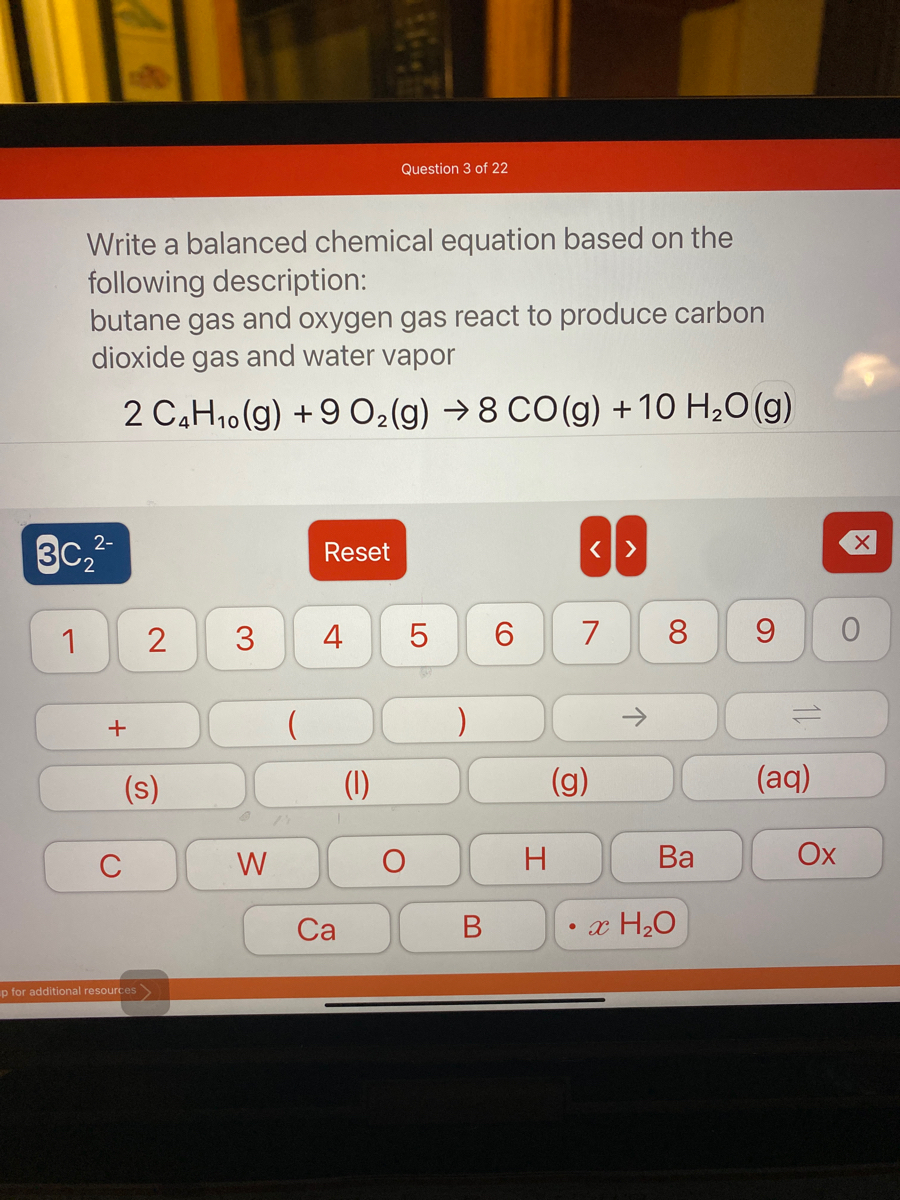
SOLVED: Gaseous butane, C4H10, reacts with diatomic oxygen gas to yield gaseouscarbon dioxide and water vapor.

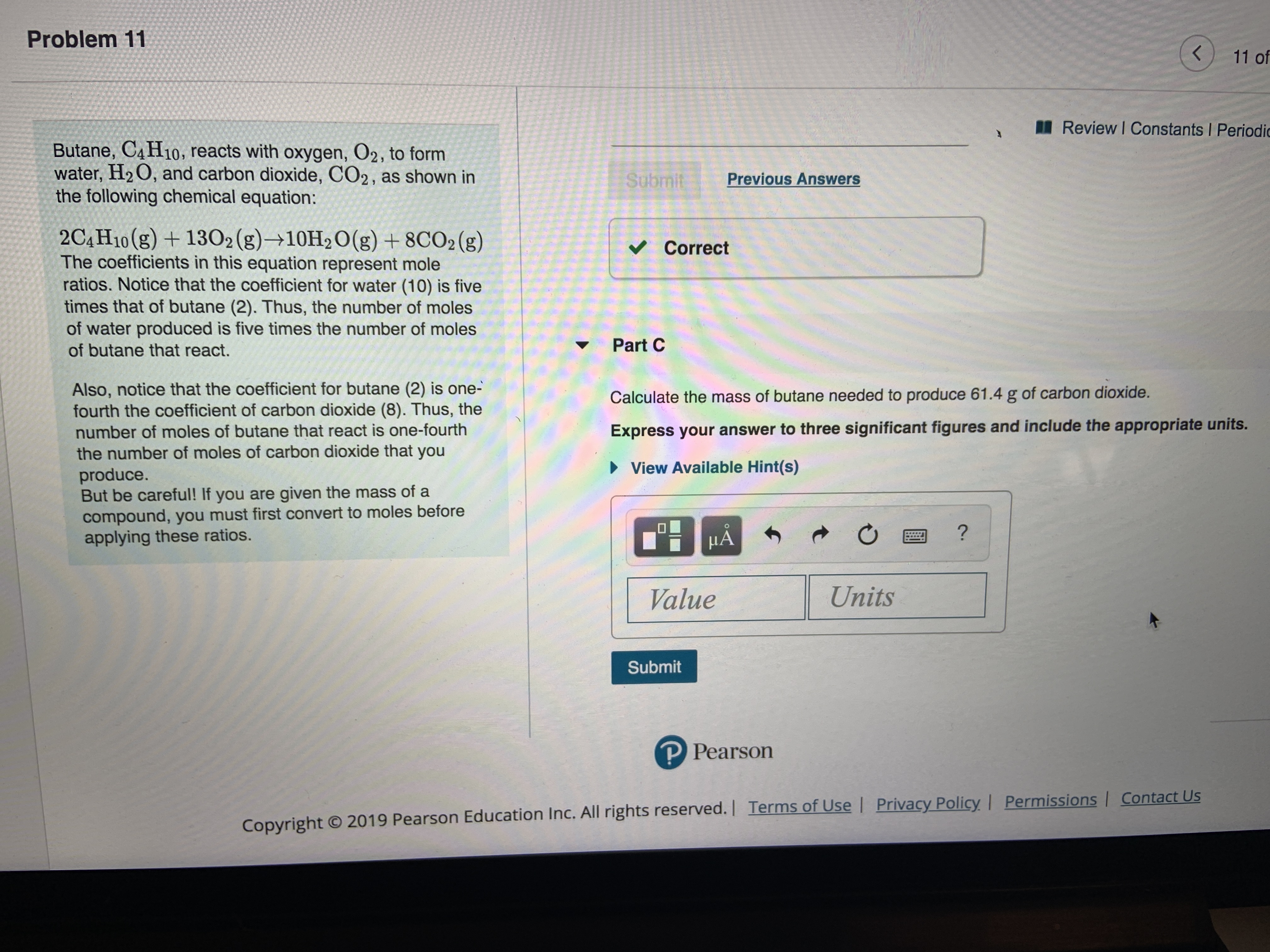
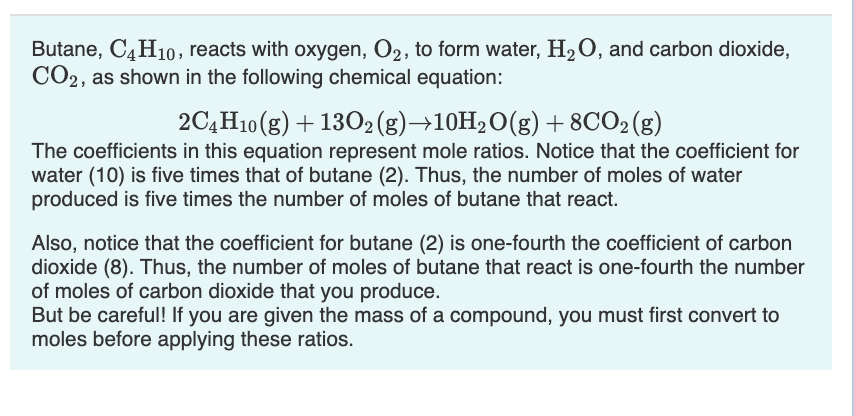
SOLVED: Butane, C4H10, reacts with oxygen, O2, to form water, H2O, and carbon dioxide, CO2, as shown in the following chemical equation: 2C4H10(g)+13O2(g)?10H2O(g)+8CO2(g) The coefficients in this equation represent mole ratios. Notice

When butane burns in oxygen, it produces carbon dioxide and water. This reaction is represented in - Brainly.com

Butane (C(4)H(10)) gas burns in oxygen to give carbon dioxide and water accoding to the reaction. 2C(4)H(10) (g) + 13O(2)(g) rarr 8 CO(2)(g) + 10H(2)O(l) When 5.0 L of butane ware burnt
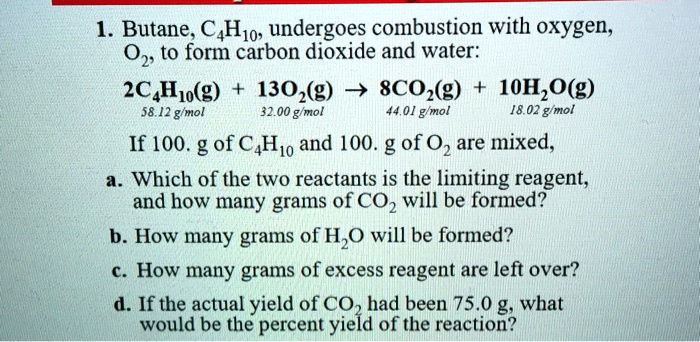
SOLVED: Butane CHzo undergoes combustion with oxygen, O1 to form carbon dioxide and water: 2C Huo(g) 130,(g) 7 8CO2(g) 10H, C O(g) 58.12 g/mol 32.00 gmol 44.01 g/mol 18.02 g mol If
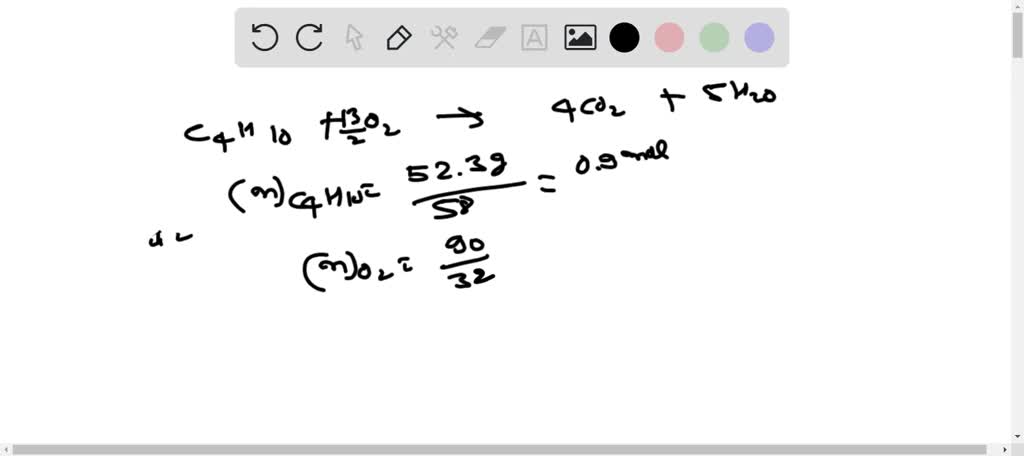
SOLVED: Gaseous butane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . Suppose 5.23 g of butane is mixed with 9.5 g of oxygen. Calculate the maximum