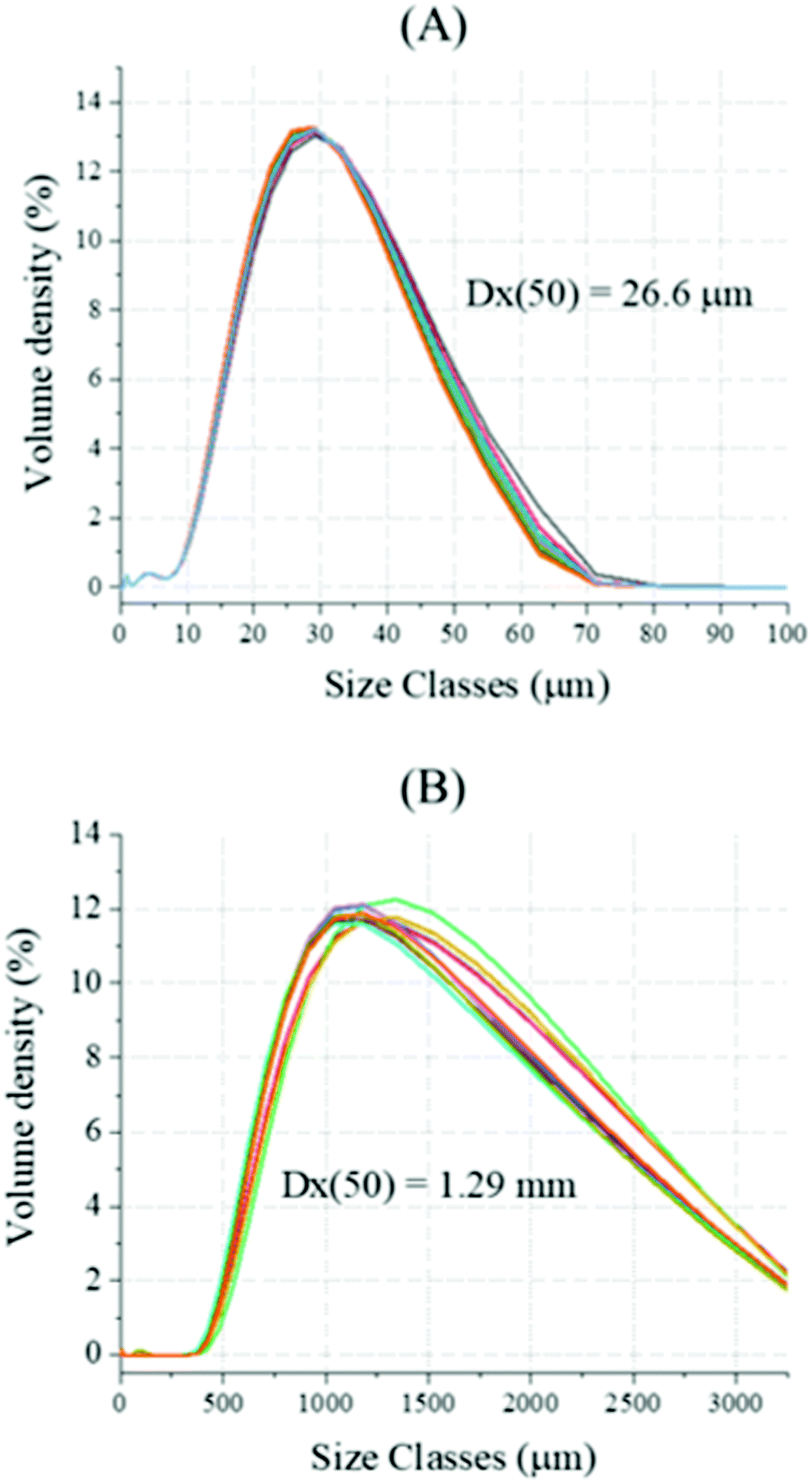
Decarbonisation of calcium carbonate in sodium hydroxide solutions under ambient conditions: effect of residence time and mixing rates - Physical Chemistry Chemical Physics (RSC Publishing) DOI:10.1039/D2CP01412B
![PDF] Regeneration of Sodium Hydroxide from a Biogas Upgrading Unit through the Synthesis of Precipitated Calcium Carbonate: An Experimental Influence Study of Reaction Parameters | Semantic Scholar PDF] Regeneration of Sodium Hydroxide from a Biogas Upgrading Unit through the Synthesis of Precipitated Calcium Carbonate: An Experimental Influence Study of Reaction Parameters | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/00e03ec2cffecd61e840107d0d9fcb645c1e1c00/3-Figure1-1.png)
PDF] Regeneration of Sodium Hydroxide from a Biogas Upgrading Unit through the Synthesis of Precipitated Calcium Carbonate: An Experimental Influence Study of Reaction Parameters | Semantic Scholar
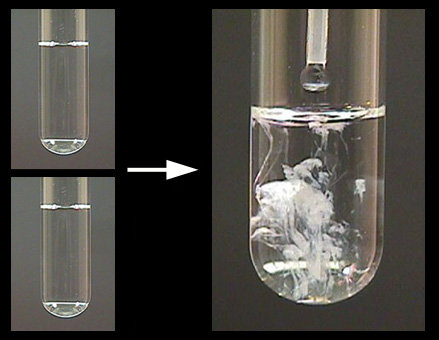
What happens when aqueous solutions of calcium chloride and of sodium carbonate are mixed? | Socratic
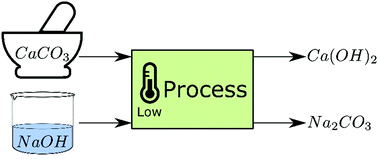
Decarbonisation of calcium carbonate at atmospheric temperatures and pressures, with simultaneous CO2 capture, through productio
Decarbonisation of calcium carbonate in sodium hydroxide solutions under ambient conditions: effect of residence time and mixing

Caustic Soda Flakes 99% Water Treatment Sodium Hydroxide Factory Price - China Potassium Hydroxide, Caustic Soda Factories | Made-in-China.com

Sodium hydroxide and sodium silicate properties which was used in this... | Download Scientific Diagram

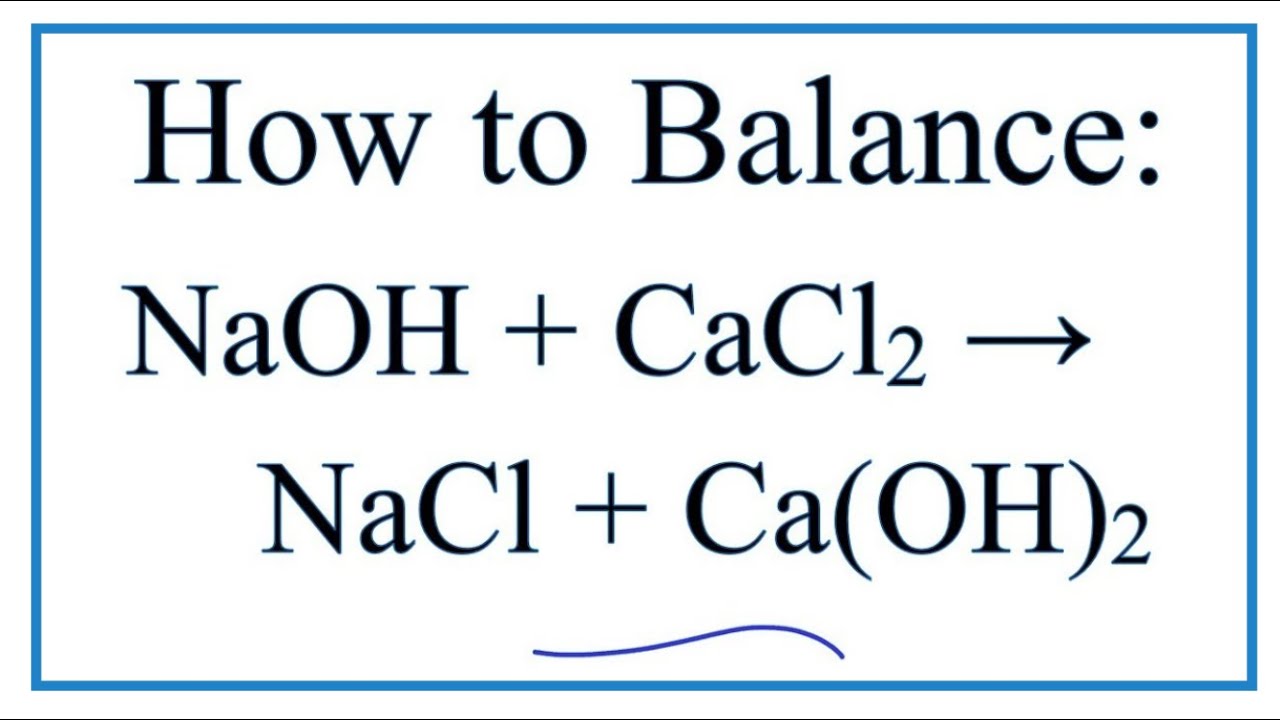
Write the balanced chemical equations for the following reactions.A Calcium hydroxide + Carbon dioxide → Calcium carbonate + waterB Zinc + Silver nitrate → Zinc nitrate + SilverC Aluminium + copper chloride

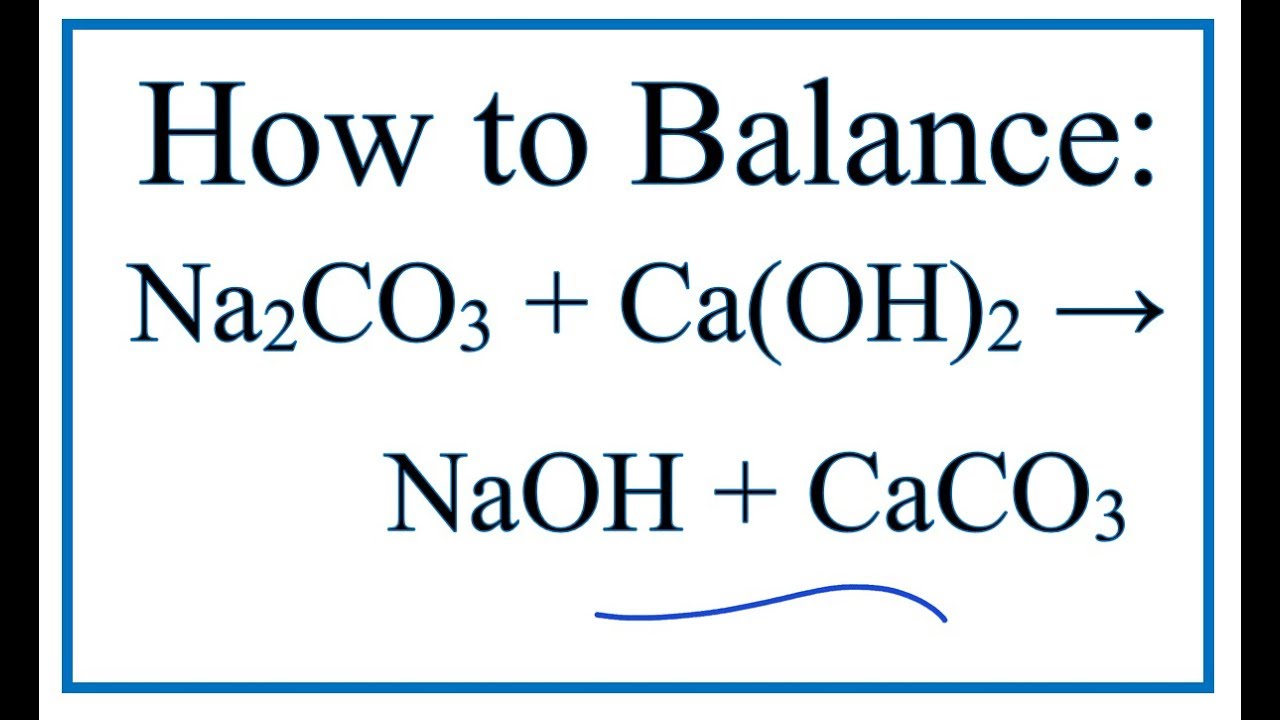
SOLVED: If 3.5 moles of sodium carbonate react with excess calcium carbonate, how many grams of sodium hydroxide will be produced? Na2CO3 + Ca(OH)2 NaOH + CaCO3
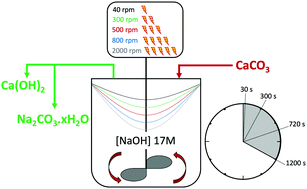
Decarbonisation of calcium carbonate in sodium hydroxide solutions under ambient conditions: effect of residence time and mixing rates - Physical Chemistry Chemical Physics (RSC Publishing)
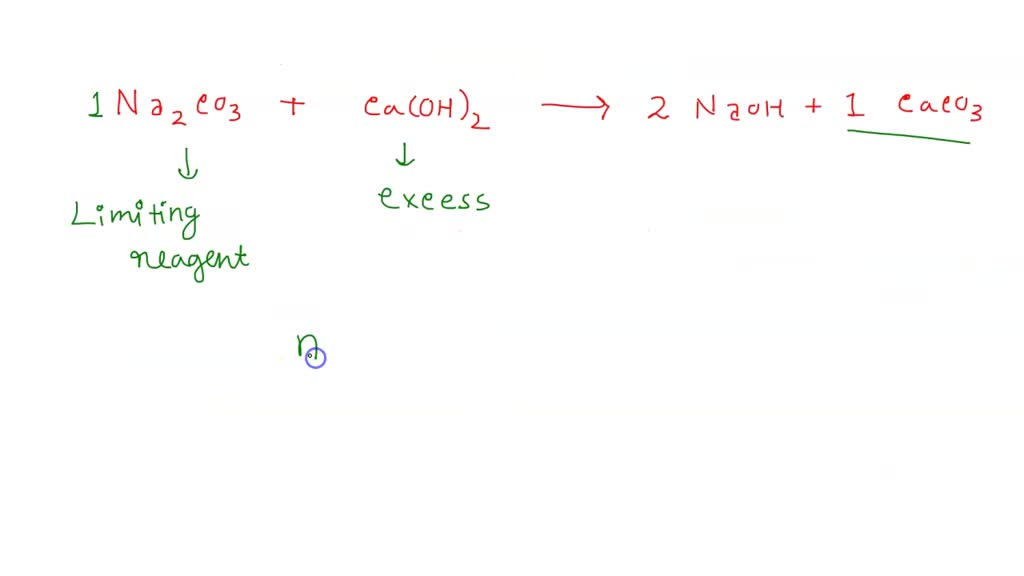
SOLVED: If 3.5 moles of sodium carbonate react with excess calcium carbonate, how many grams of sodium hydroxide will be produced? Na2CO3 + Ca(OH)2 NaOH + CaCO3

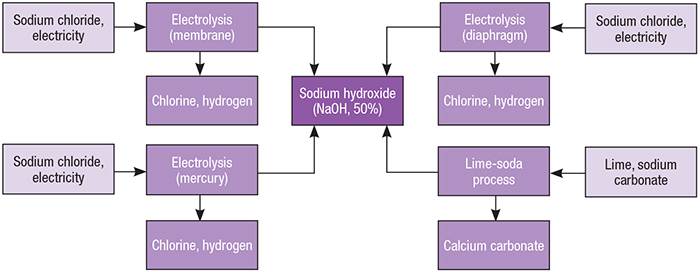
Figure 3 from Recovery of Sodium Hydroxide from Trona Ore and Calcium Carbonate as Raw Materials | Semantic Scholar
Decarbonisation of calcium carbonate in sodium hydroxide solutions under ambient conditions: effect of residence time and mixing
![PDF] Recovery of Sodium Hydroxide from Trona Ore and Calcium Carbonate as Raw Materials | Semantic Scholar PDF] Recovery of Sodium Hydroxide from Trona Ore and Calcium Carbonate as Raw Materials | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/c111a7b476ee6999fdd843657756ea6a4f670b83/3-Figure1-1.png)
PDF] Recovery of Sodium Hydroxide from Trona Ore and Calcium Carbonate as Raw Materials | Semantic Scholar

Bases. Jars containing calcium carbonate (Ca2CO3), copper oxide (CuO) and sodium hydroxide (NaOH). These compounds are classified as bases, because th Stock Photo - Alamy