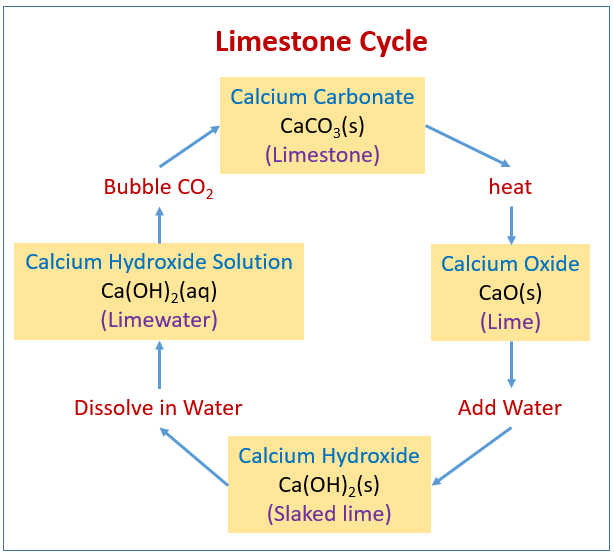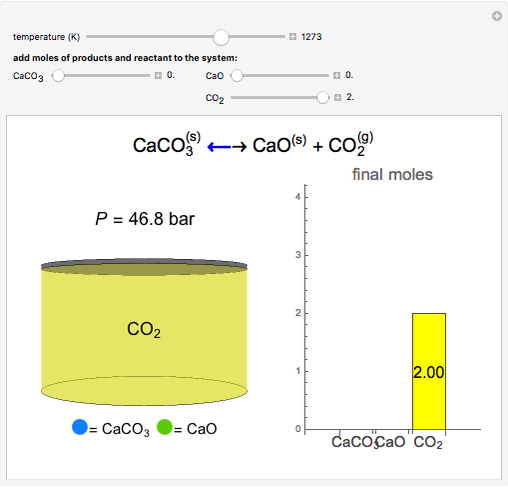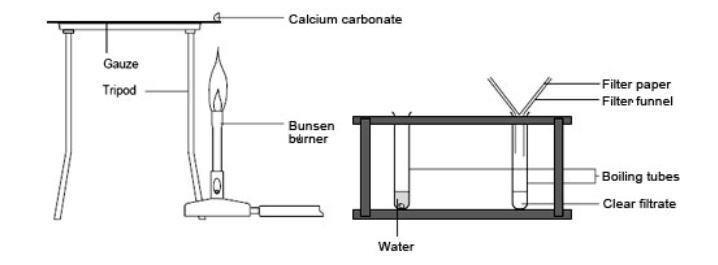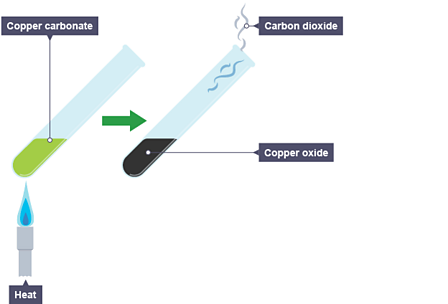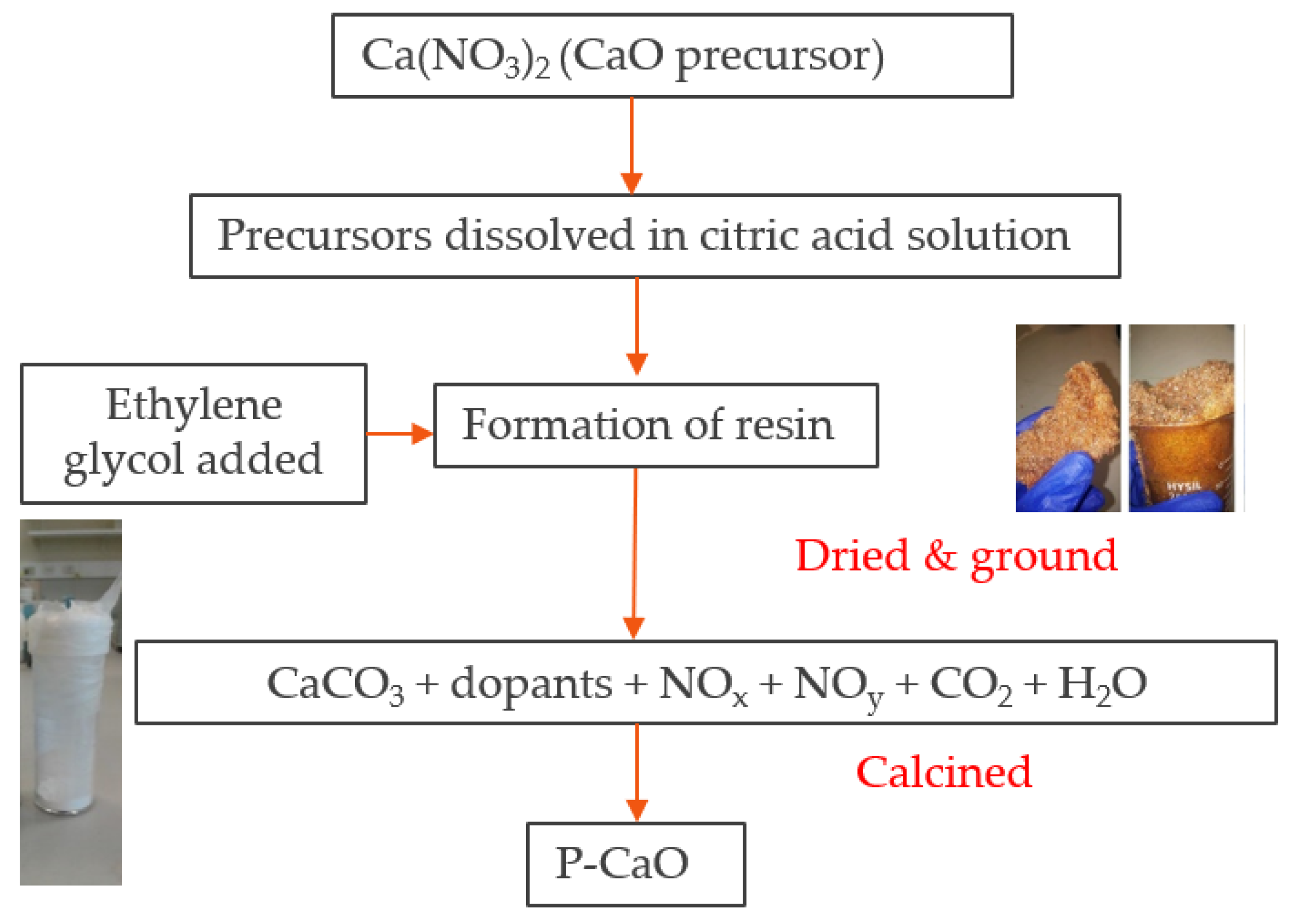
Applied Sciences | Free Full-Text | Comparative Kinetic Analysis of CaCO3/CaO Reaction System for Energy Storage and Carbon Capture
Mortar is made using limestone, which is composed primarily of calcium... | Download Scientific Diagram
Write balanced chemical equation for the following processes: (a) heating calcium in oxygen (b) heating calcium carbonate - Sarthaks eConnect | Largest Online Education Community

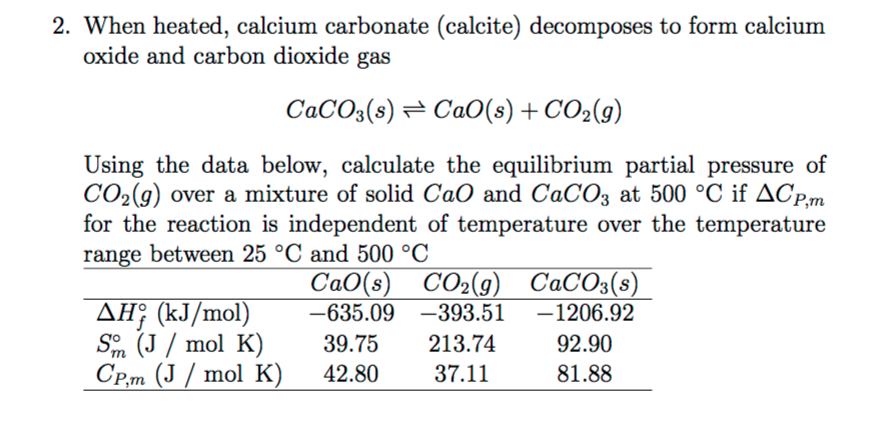
Thermal decomposition of calcium carbonate (calcite polymorph) as examined by in-situ high-temperature X-ray powder diffraction - ScienceDirect

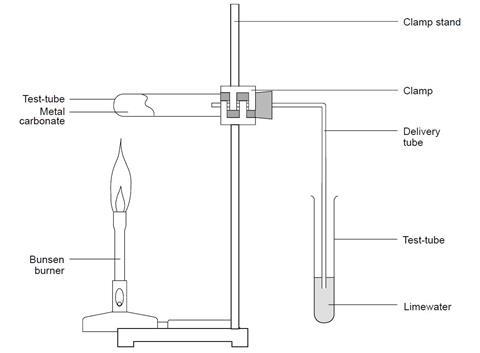
Carbon Dioxide from Thermal Decomposition (2.3.4) | Edexcel IGCSE Chemistry Revision Notes 2019 | Save My Exams
The calcium carbonate reaction ratio and heating curve of the solution... | Download Scientific Diagram

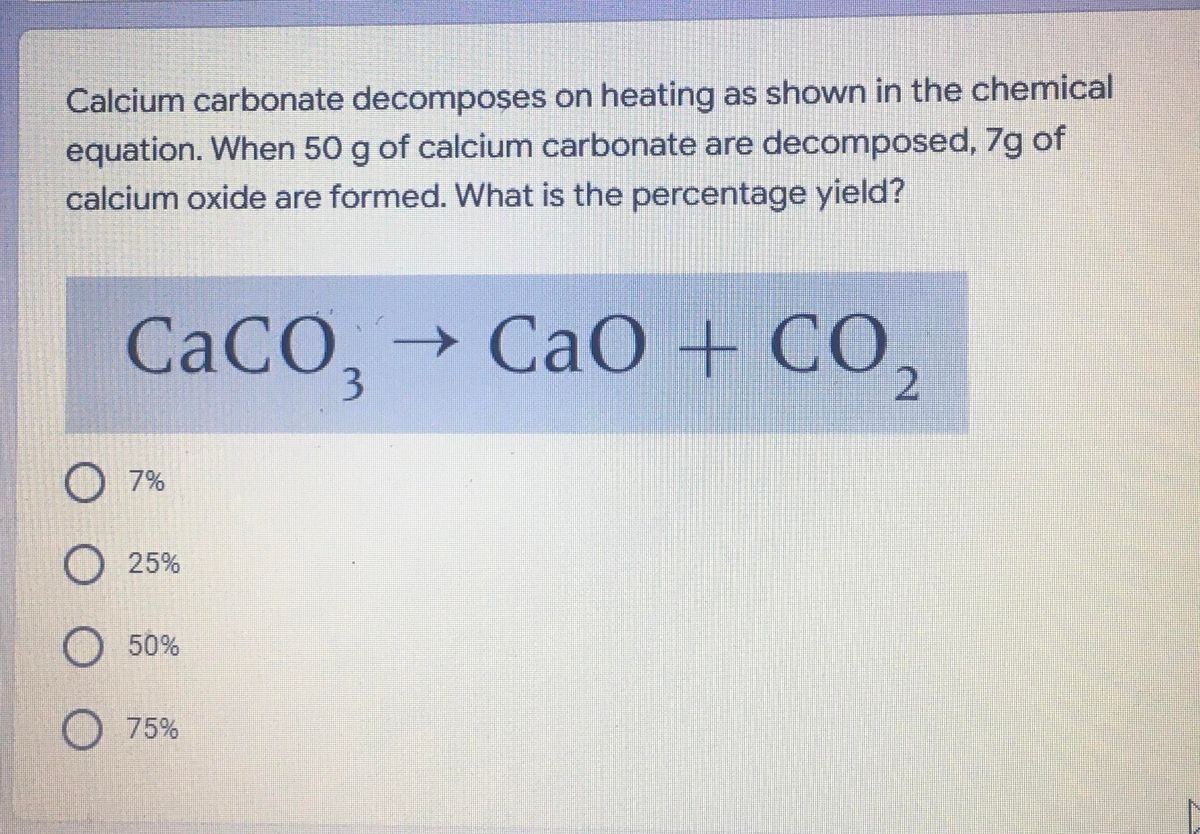
50 g of an impure calcium carbonate sample decomposes on heating to give carbon dioxide and 22.4 g calcium oxide. The percentage purity of calcium carbonate in the sample is:
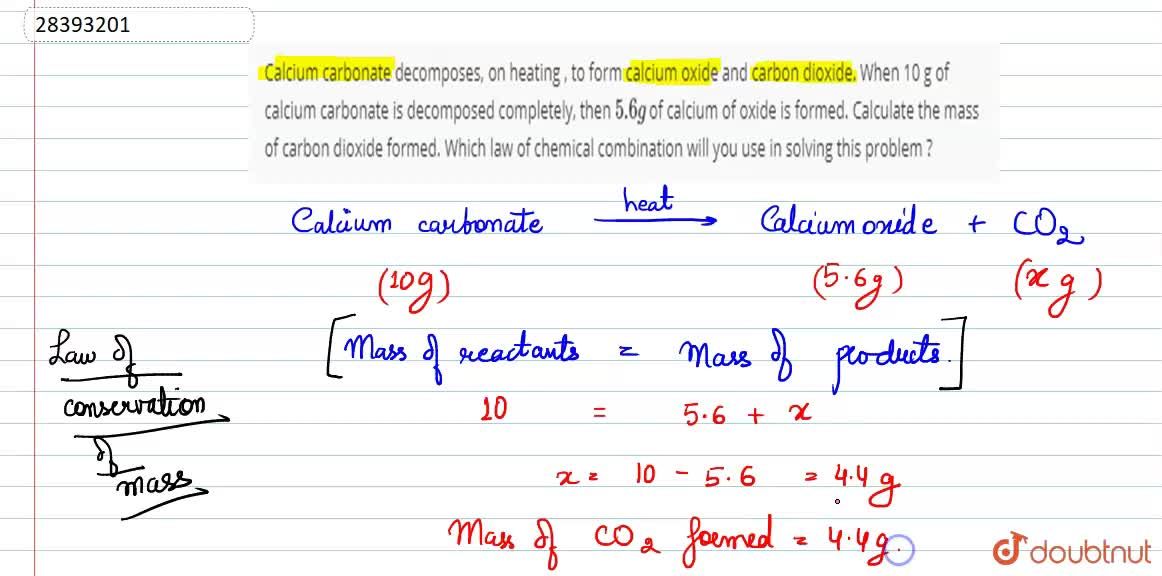
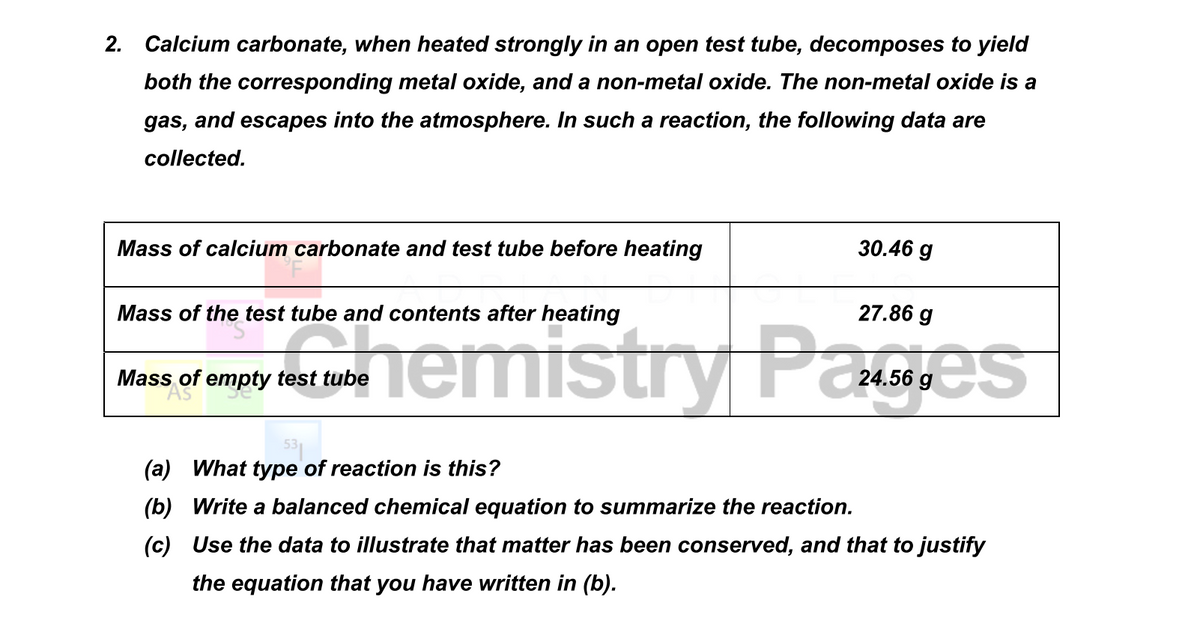
Calcium carbonate decomposes, on heating , to form calcium oxide and carbon dioxide. When 10 g of calcium carbonate is decomposed completely, then 5.6 g of calcium of oxide is formed. Calculate