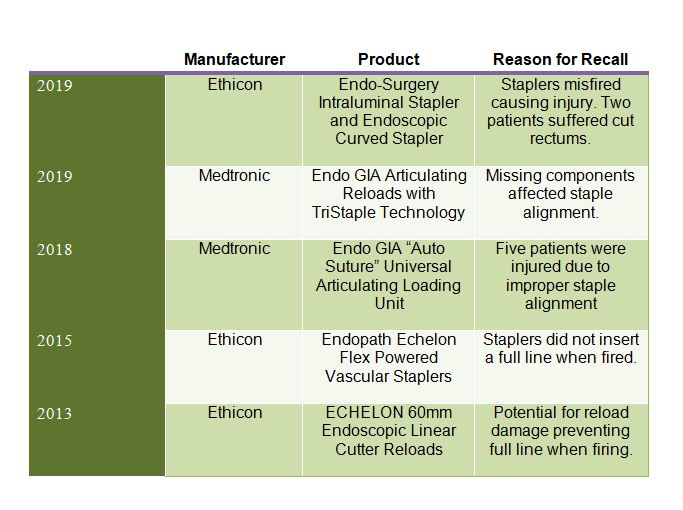
Ethicon Stapler Recall Removes Over 90,000 Units From Circulation | MedTruth - Prescription Drug & Medical Device Safety | Informed Advocacy
Ethicon Circular Stapler Recall Issued Over Insufficient Firing Events, Injury Risks - AboutLawsuits.com
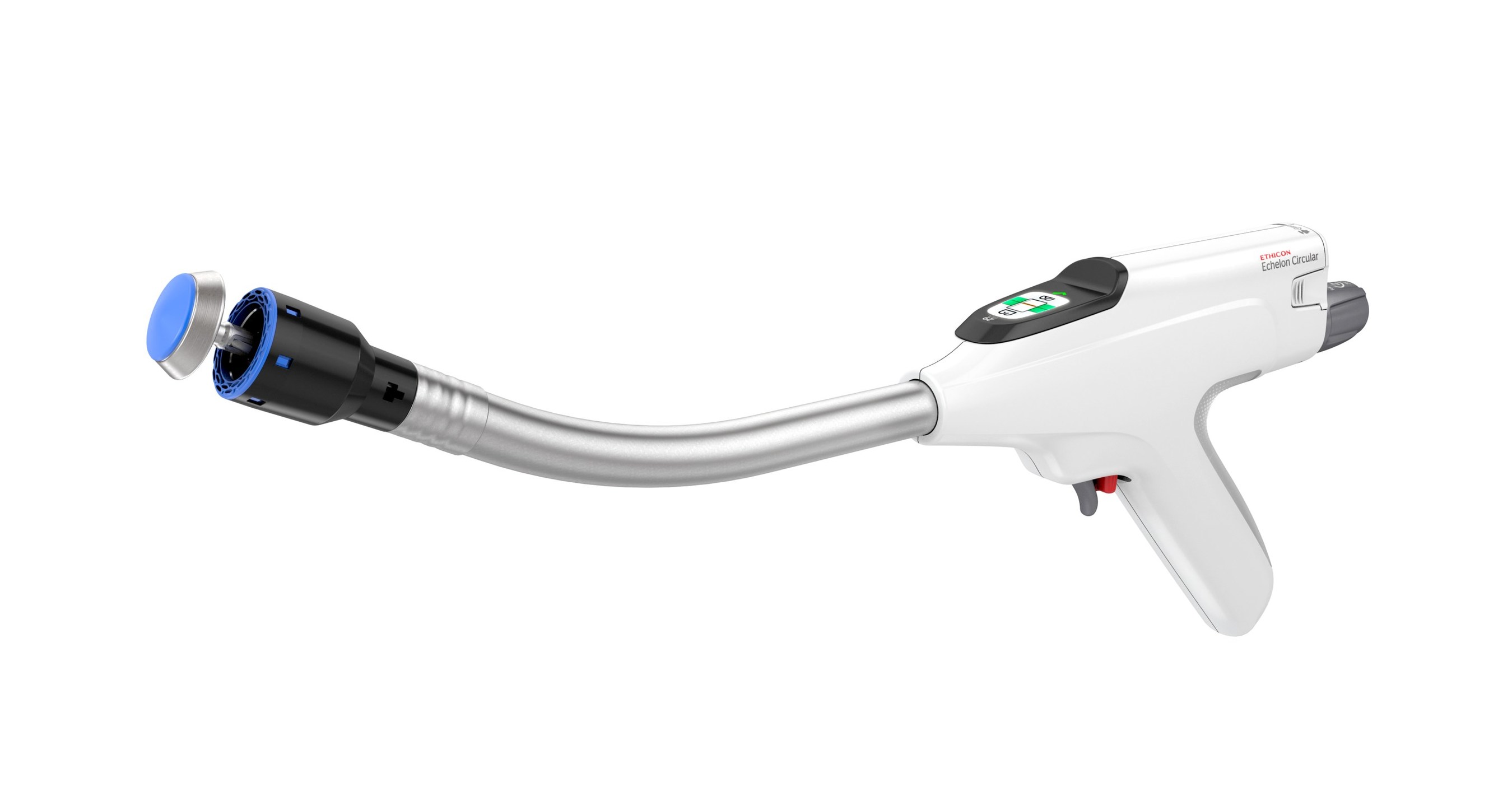
Ethicon's Echelon Circular Powered Stapler Associated With Major Reduction In Serious Complications Following Colorectal Surgery