UAEM | Clinical Trials Transparency - The role of universities in a more equitable biomedical R&D system
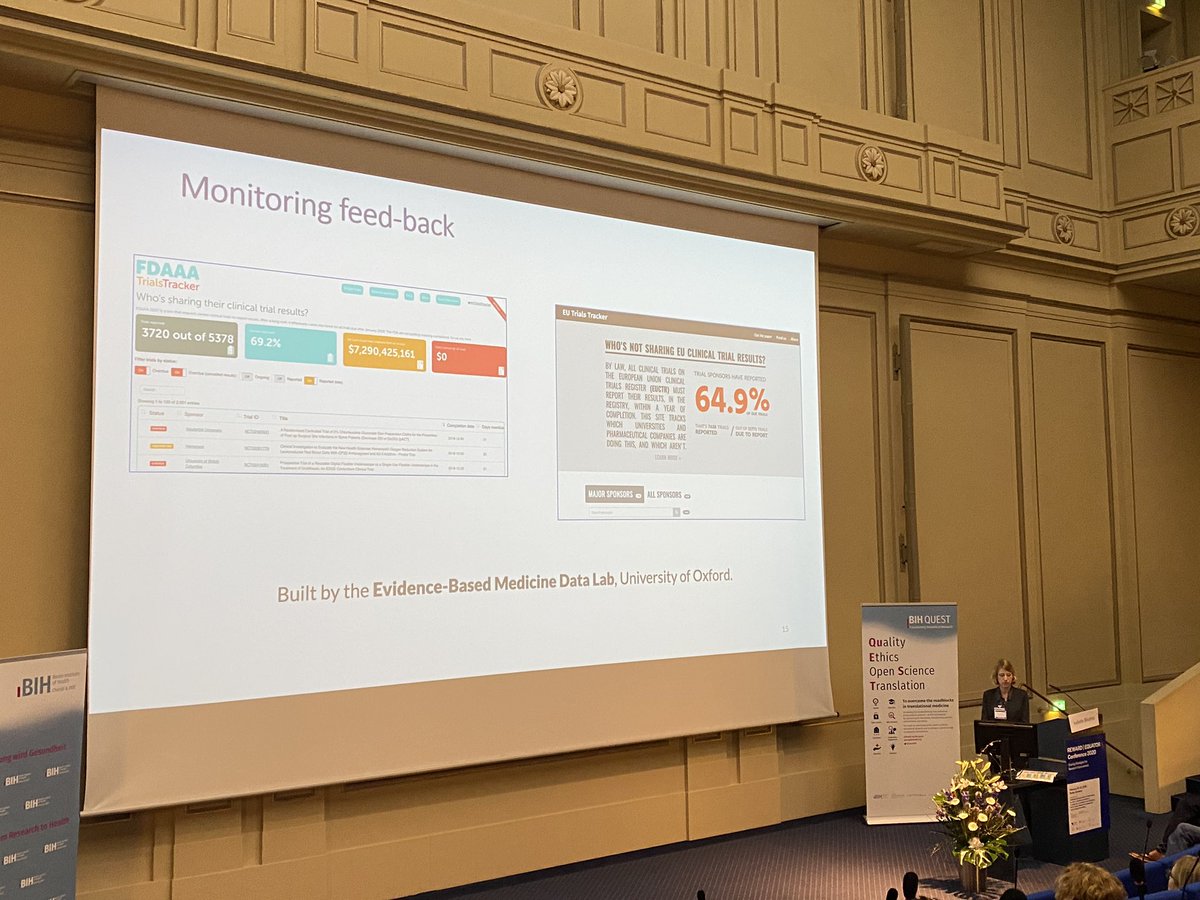
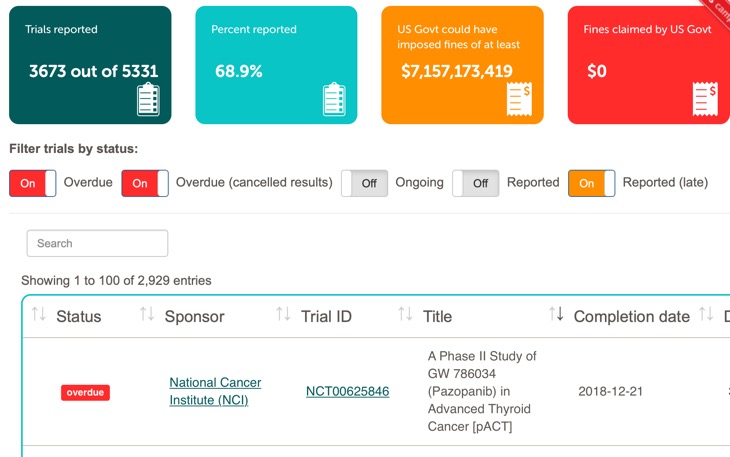
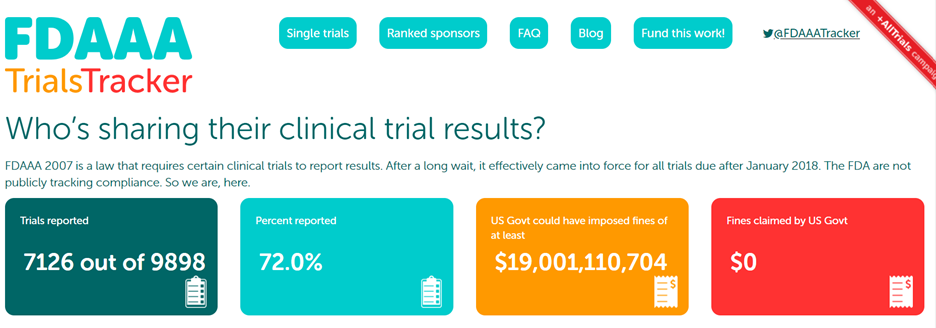
FDAAA TrialsTracker: A live informatics tool to monitor compliance with FDA requirements to report clinical trial results

FDAAA TrialsTracker: A live informatics tool to monitor compliance with FDA requirements to report clinical trial results | bioRxiv

FDAAA Trials Tracker: leaderboard for pharma companies that break FDA clinical trial rules - Boing Boing
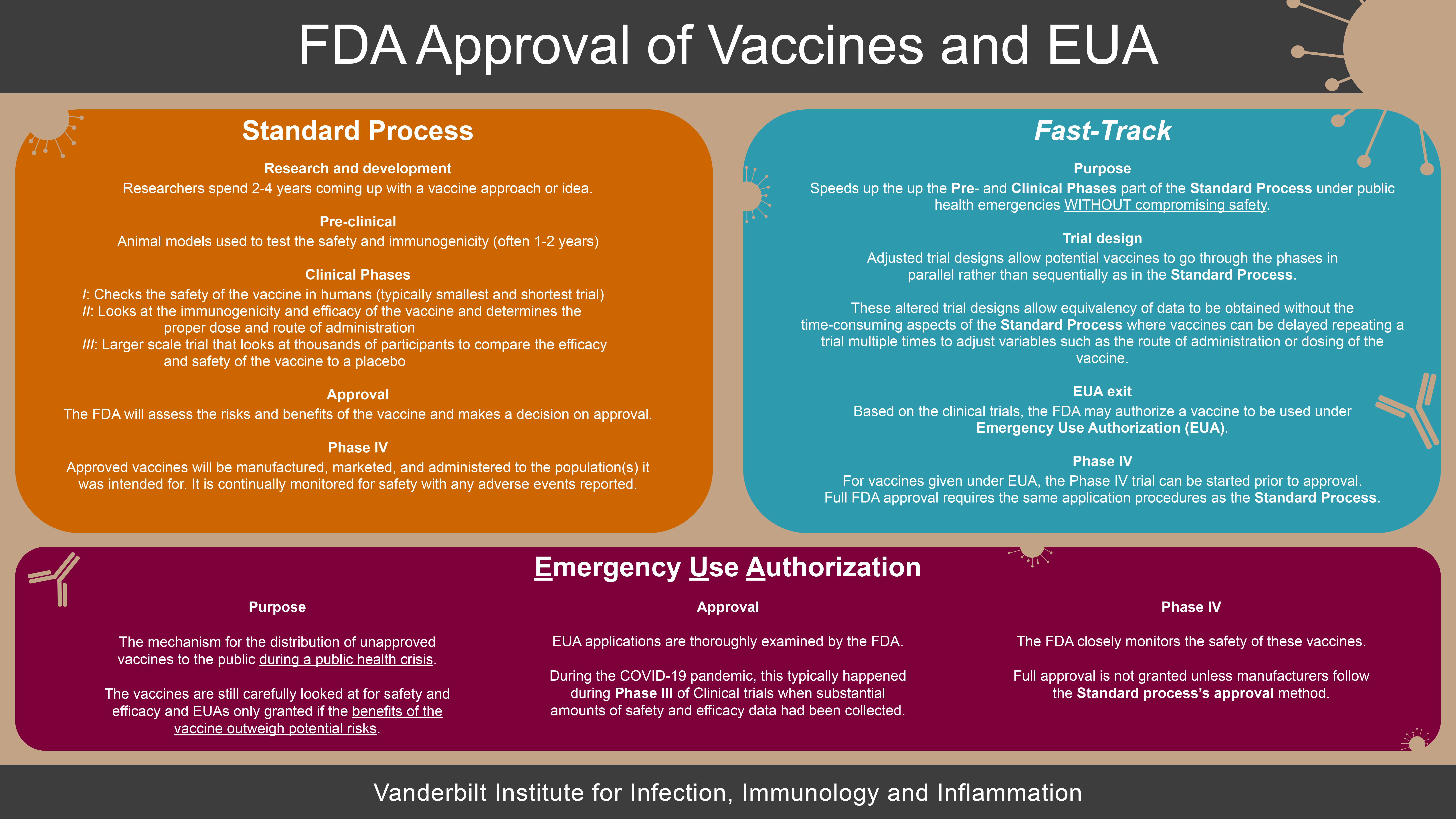
FDA Approval of Vaccines and EUA Infographic | Vanderbilt Institute for Infection, Immunology and Inflammation
What proportion of clinical trial data is reported? TrialsTracker launches new monitoring tool – The Publication Plan for everyone interested in medical writing, the development of medical publications, and publication planning