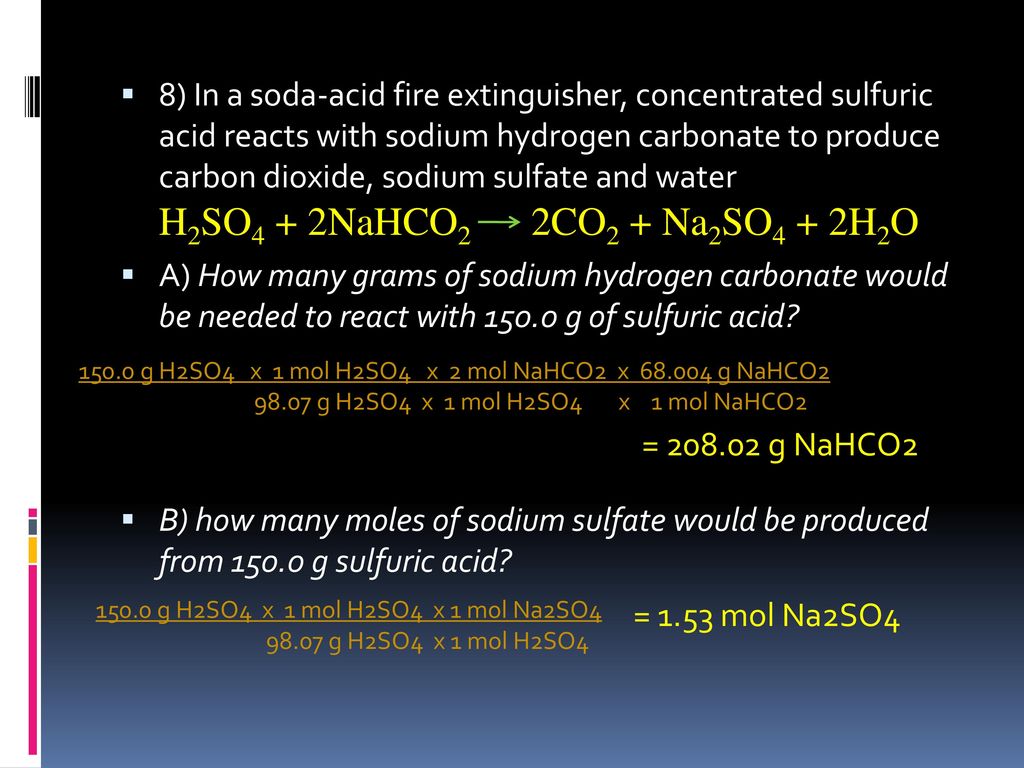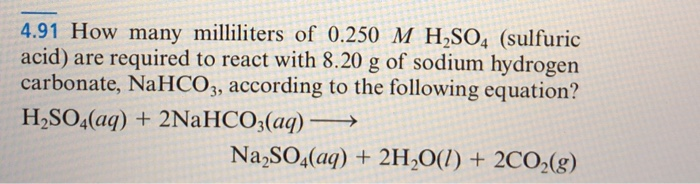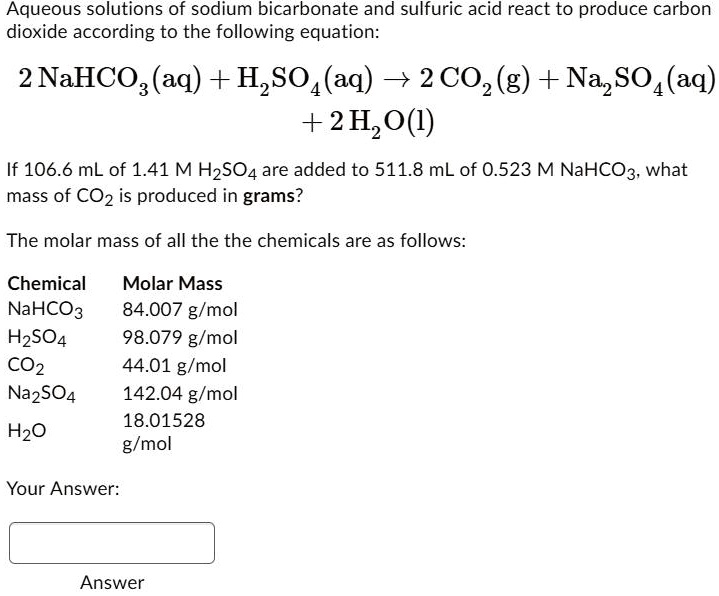
SOLVED: Aqueous solutions of sodium bicarbonate and sulfuric acid react to produce carbon dioxide according to the following equation: 2 NaHCO3(aq) + H,SO4(aq) = 2 C02(g) + Na2 SO4(aq) +2H20(1) If 106.6

The graph of moles of sodium hydrogen carbonate versus moles of sulfuric acid produce a linear graph with the equation y = 2x - 2E-17. The molar mass of sodium hydrogen carbonate
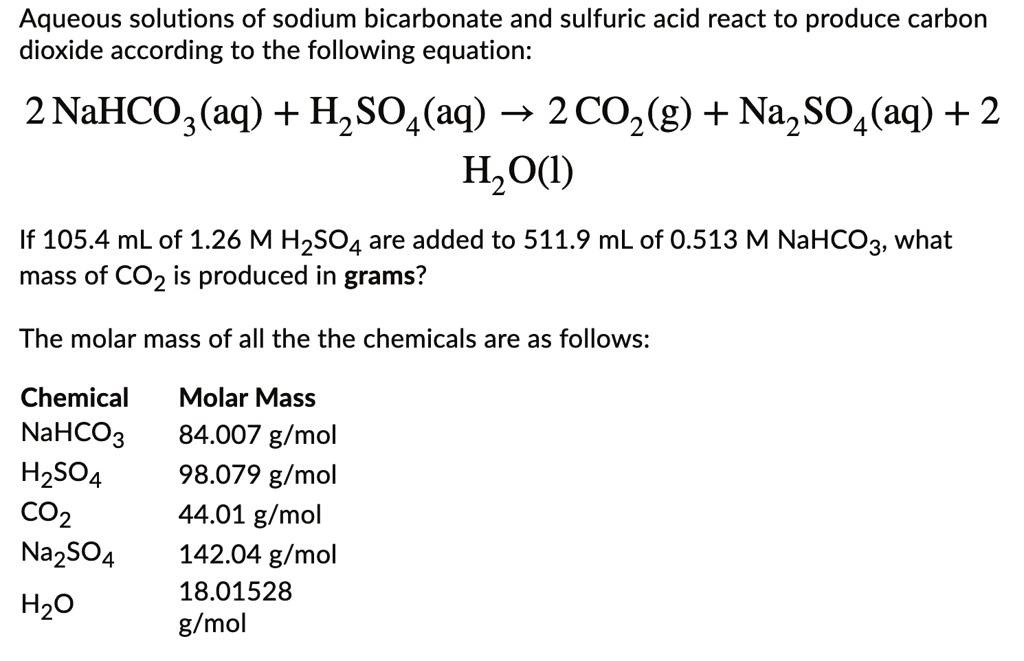
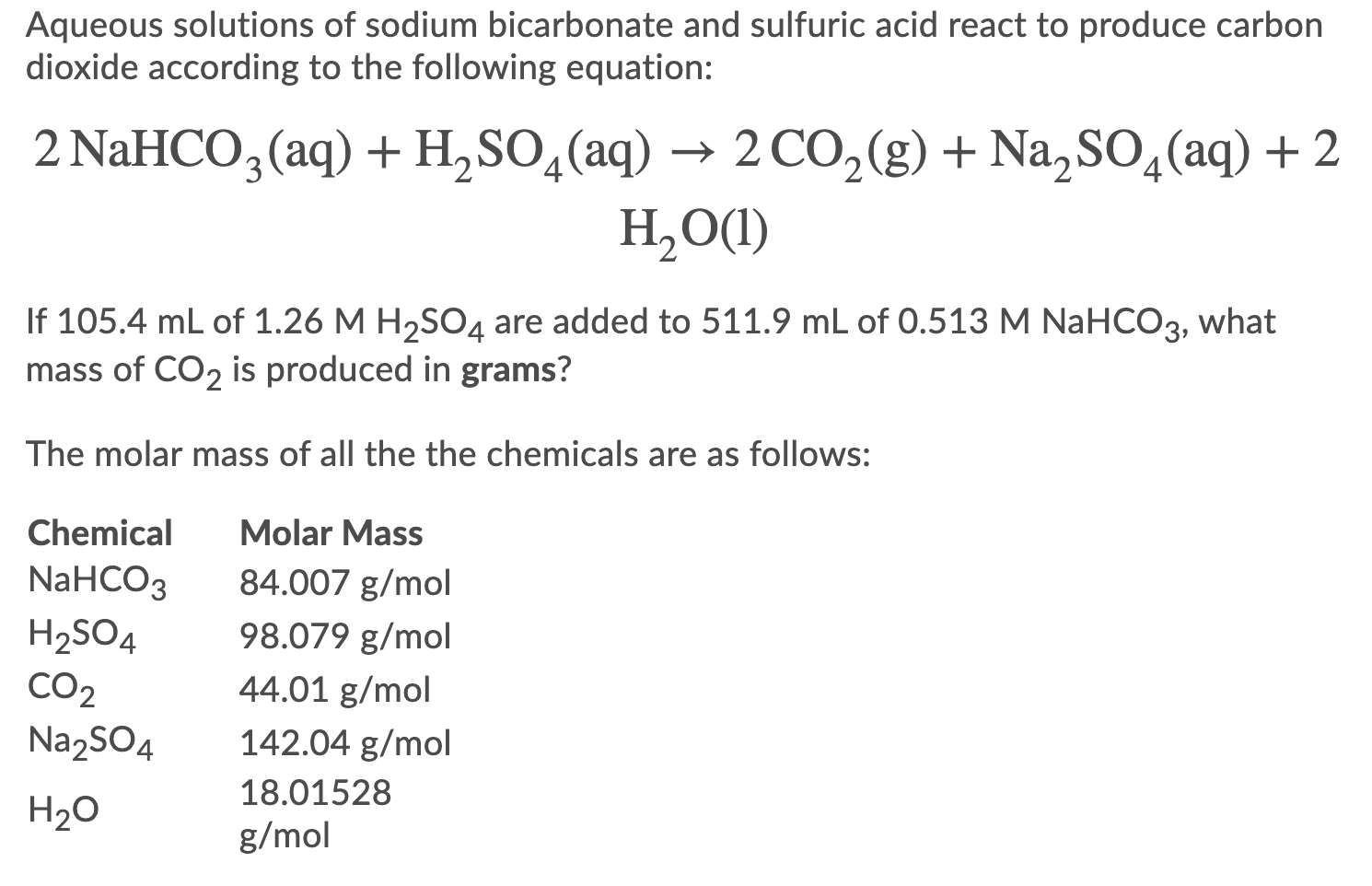
SOLVED: Aqueous solutions of sodium bicarbonate and sulfuric acid react to produce carbon dioxide according to the following equation: 2 NaHCOs(aq) + H,SO4(aq) 3 2CO2(g) + NazSO4(aq) + 2 HzO() If 105.4

Question Video: Determining the Concentration of Sulfuric Acid Via Titration with Sodium Carbonate | Nagwa

Question Video: Identifying the Volatile Compound in a Reaction for Volatilization Gravimetry | Nagwa

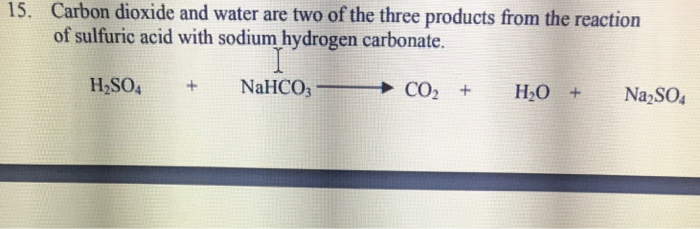
balance the equation Sodium bicarbonate + Sulphuric acid = Sodium sulphate + Water + Carbon dioxide. - Brainly.in
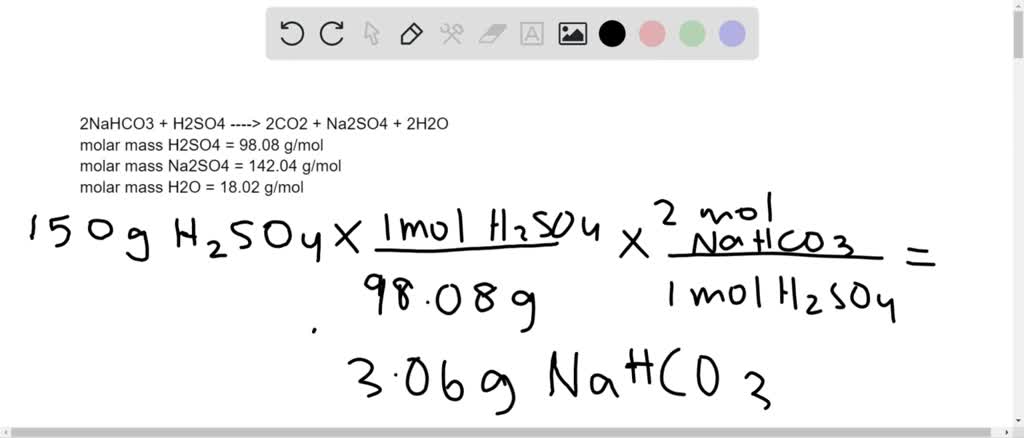
SOLVED:In a soda-acid fire extinguisher, concentrated sulfuric acid reacts with sodium hydrogen carbonate to produce carbon dioxide, sodium sulfate, and water. a. How many moles of sodium hydrogen carbonate would be needed